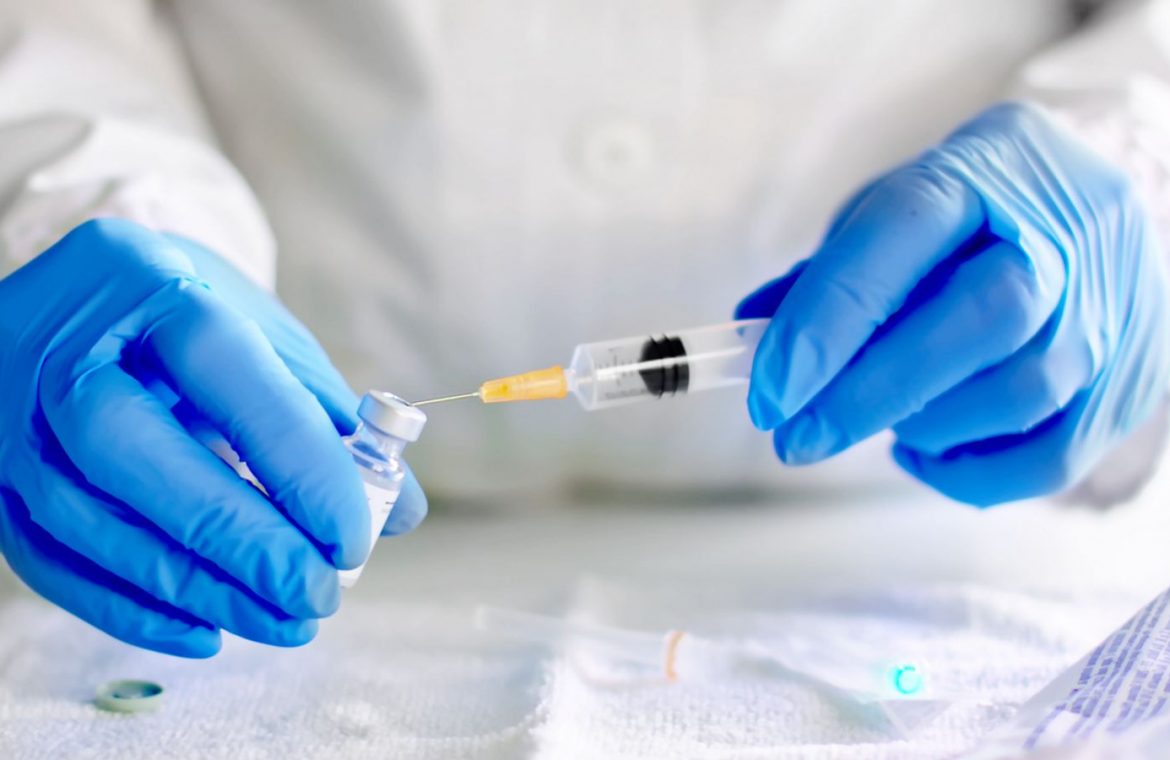
The British volunteers will be deliberately infected with the COVID-19 virus to test whether the vaccine offers any protection.
In the first trial of its kind, participants will be injected with an experimental vaccine and about a month later they are exposed to Sars-Cov-2, the virus that causes the disease.
According to FT, The studies – known as human challenge trials – will start in January with government funding.
Live updates on Coronavirus from the UK and around the world
The experiments are reported to be held at a secure facility in Whitechapel, east London.
A government spokesperson said it is looking at collaborating in the potential development of a vaccine through human challenge studies.
They added that “these discussions are part of our work to discuss methods of treating, limiting and preventing the virus, in the hope that we will be able to end the epidemic soon.”
Any experiment involving exposing people to the virus will need approval from the UK Medicines and Healthcare products Regulatory Agency (MHRA), as well as an independent research committee.
Challenge trials are controversial.
Even young adults are at very little risk of contracting a serious illness from the virus and some doctors believe this is against medical ethics.
There is also a risk that they may experience symptoms of ‘prolonged COVID’ similar to chronic fatigue syndrome.
:: Subscribe to the daily podcast on Apple Podcast, Google Podcast, Spotify, speaker
But as levels of virus spread in the population continue to decrease, trials are the fastest way to test the level of protection from a vaccine.
Scientists need to know whether the vaccines being developed prevent people from contracting the virus, or whether they only eliminate symptoms.
Dr. Claire Waddington, clinical lecturer in infectious diseases at the University of Cambridge, said the challenge trials “are well established as a way to accelerate vaccine development.”
She pointed to similar experiences used in typhoid vaccines, which are now being rolled out in affected countries.
“As we gain more understanding of COVID-19, we are increasingly able to identify people for whom COVID-19 infection is a mild disease, and these people can safely participate in a controlled human infection study after a comprehensive medical evaluation and approval process,” she said.
“Such a model could give us some very useful information about how the immune system is responding to COVID and what protective responses are, in addition to providing a template for early testing of candidate vaccines.”
The MHRA said: “The safety of trial participants is our top priority and any proposal by a developer to include a human infection challenge as part of a clinical trial to develop a vaccine will be considered on the basis of risk of benefit, with the risks monitored in order to design the proposed trial and minimize it.”

“Proud creator. Amateur music junkie. Tv scholar. Web fan. Lifelong alcohol lover. Falls down a lot. Hardcore thinker.”